
Think about the last time you picked up a prescription from the pharmacy. If you read the information in the package insert, you may have assumed that the data about reported side effects and other patient outcomes was all drawn from randomized controlled trials. This may not be the case. Instead, you may have been reaping the benefits of a form of qualitative research output known as real-world evidence (RWE).
In the webinar Coding the Real World: What Is Real-World Evidence in Health and Why Is NVivo Critical?, three senior researchers from Cerner Enviza, an Oracle Company, explained why RWE is increasingly critical in drug development and healthcare research. They also described how using coding software for qualitative research like NVivo helps generate robust analyses from unstructured data that communicate insights to research teams, journal publishers, regulators, and commercial stakeholders.
Representing Cerner Enviza were:
- Kathleen Beusterien, MPH – Principal, RWE
- Colleen Welsh-Allen, RN – Qualitative Research Subject Matter Expert in the U.S. for Commercial, RWE, and Regulatory
- Rebecca Nash, PhD – Business Leader, RWE
In this article, we’ll highlight the main discussion points – from their research methods to using NVivo coding software for qualitative data analysis or QDA.
Defining Real-World Evidence and Its Applications in Healthcare Research
“If you're like me, a qualitatively trained social scientist,” said Dr. Rebecca Nash, “the term ‘real world’ seems a little bit funny, because isn't everything the real world?” However, she explained, RWE within the healthcare and pharmaceutical industries simply means any evidence that isn’t gathered within the context of a randomized controlled trial. RWE can also mean any evidence that isn’t generated by a lab test or imaging technique, such as a blood analysis or MRI.
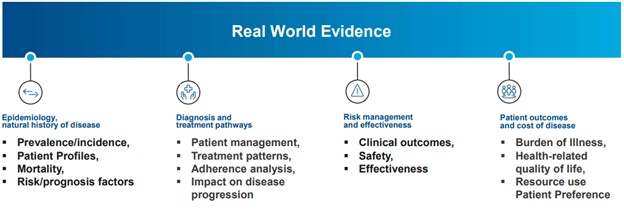
Real world evidence examples in the areas of epidemiology, natural history of disease, diagnosis and treatment pathways, risk management and effectiveness, and patient outcomes, and cost of disease.
Usually, RWE is gathered through qualitative interviews focused on understanding patient preferences or health outcomes – any change in their status following a treatment or procedure. “Rigorous qualitative analysis is so important in bringing forth the voice of the patient,” said Kathleen Beusterien.
Insights drawn from RWE complement randomized controlled trials and help inform drug development at every stage. RWE can influence clinical trial design, regulatory compliance reporting, treatment or diagnosis pathways, and marketing. It also informs the prescription medication inserts that explain side effects which can’t be measured with lab tests like pain, fatigue, or symptoms of depression.
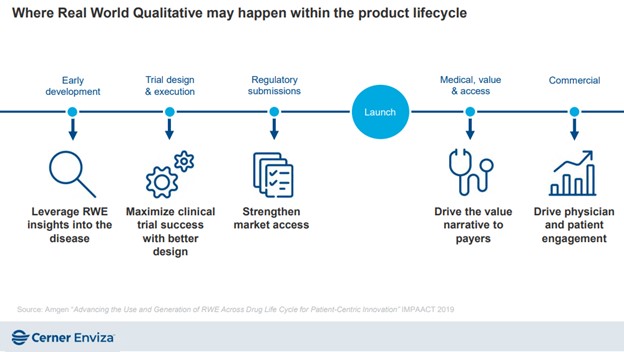
Alt text: Timeline showing where real world qualitative data and research may happen within the product lifecycle. Phases before the launch include early development, trial design and execution, regulatory submissions and after the launch include medical, value, and access, and commercial.
The Cerner Enviza team presented three examples of how RWE helped researchers understand patient needs and experiences during drug development:
- Interviews with the families of pediatric patients coping with a rare genetic disorder called neurofibromatosis type 1 gave researchers insight into the symptoms that potential therapies could help treat.
- RWE from parents of children with severe allergies helped researchers develop an interactive diagnosis pathway that explained how clinicians can help families move from the chaos of an initial attack to informed self-management of their child’s condition.
- After conducting qualitative research, development teams were better able to understand why diabetes patients preferred the nasal spray form of glucagon – a drug that stops potentially fatal hypoglycemic episodes – rather than the injectable form.
Qualitative Analysis and Research Methodologies for Gathering RWE
The Cerner Enviza team explained that while the FDA and other regulators increasingly accept or even require RWE about patient preferences or health outcomes, there are guidelines about how to conduct this kind of qualitative research. There are two main methodologies for capturing RWE in the healthcare or pharmaceutical context.
First, there is concept elicitation (CE). CE involves an open-ended, narrative-style one-to-one interview. “It's a fancy name for qualitative interview,” said Colleen Welsh-Allen. She went on to explain that these interviews can be conducted over the phone, in person, via an internet video tool such as Zoom, or in an online chat. In addition to yielding insight into patient needs and experiences, CE can also help inform the development of survey questions for quantitative studies.
The second methodology for gathering RWE is a cognitive interview. Cognitive interviewing as a methodology was developed in the 1980s, and is often required by federal agencies as a step in the development of a quantitative survey. In a cognitive interview, “we really are hoping to understand how a subject arrives at their answer,” explained Colleen Welsh-Allen. Researchers will ask patients to answer a question while explaining their reasoning. “We want to make sure that everybody has universal understanding of the intent of the question,” Welsh-Allen clarified.

After conducting qualitative research, it’s time to analyze it. That’s where NVivo comes in.
Coding in NVivo for Qualitative Research
Because healthcare and pharmaceutical research is subject to institutional review boards (IRBs, or research ethics committees), it’s required to have verbatim transcripts for all interviews conducted, to obtain counts, and to carry out rigorous coding of interviews. This may not always be required for qualitative research in other fields, but where necessary, it can be incredibly helpful to employ a qualitative data analysis tool for transcription, data visualization, and to analyze data in large quantities.
While it’s possible to analyze qualitative research in a variety of ways – for example, through discourse analysis or grounded theory methodologies – the Cerner Enviza team uses thematic analysis because it allows for a bottom-up approach that lets patient concerns or experiences emerge from the data.
NVivo’s coding features allow for robust thematic analysis and speeds up the coding process with user-driven machine-powered autocoding. This automated tool for qualitative coding of textual data saves valuable time and can reveal themes and sentiments that might otherwise have gone unnoticed.
With NVivo, RWE researchers can:
- Tease out repeated patterns and construct themes by analyzing qualitative interview transcripts.
- Indicate whether qualitative data is spontaneous/unaided versus prompted from interviewer questions.
NVivo can also generate a range of visualizations, such as saturation grids, which help analysts determine whether follow-up interviews may be necessary. Plus, with powerful NVivo Transcription, researchers can import audio and video files to produce verbatim transcriptions with 90% accuracy.
The Cerner Enviza team was also able to work together efficiently with the help of NVivo Collaboration Cloud. With this tool, they could securely share data and insights to the same project and update, code, and analyze research in real-time.
NVivo’s rigor and adaptability makes it possible for research that includes RWE to meet the standards of medical and academic journals, conference organizers, and regulators. With text analysis, content analysis, and sentiment analysis supported by NVivo, teams can gather RWE that gives patients a voice in the treatments and interventions that can advance healthcare.
Learn More About RWE and NVivo Qualitative Data Analysis Software (QDA Software)
Interested in watching the full presentation on using coding software for qualitative research and RWE in drug development? Fill out the form on this page to access the full webinar recording and handout materials.
Start exploring what’s possible in your research project with NVivo by requesting a 14-day free trial of the most cited qualitative analysis tool today.
